Understanding the clinical trial process
Understanding the clinical trial process

Clinical trials are medical studies that look at potential new medicines, procedures and other treatments in people affected by diseases such as cancer. Throughout this process, doctors try to determine whether these potential new treatments are safe and effective. Sometimes, they also determine if these treatments will help improve the quality of life for people living with cancer. Participation by cancer patients in clinical trials helps add to medical and scientific knowledge about cancer and can result in improved current and future care.
What are Clinical Trials?
Clinical trials are the final step in a long process that starts in the lab. Before any new treatment is given to people, researchers often spend many years understanding its effects through early testing. During these tests, researchers try to learn as much as they can about how the treatment works in cells and animals.
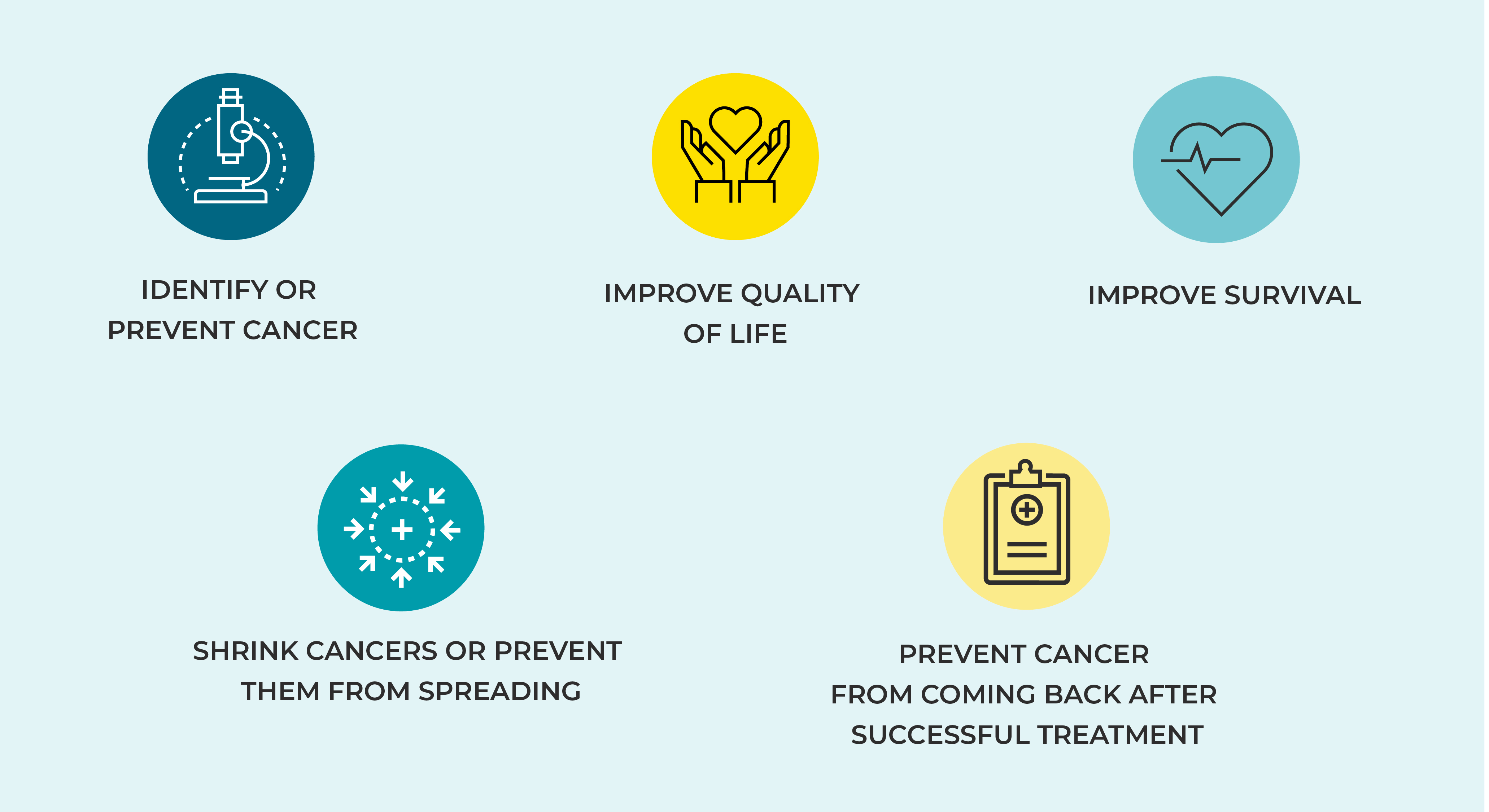
Researchers plan each cancer clinical trial to test specific questions. They may be looking for ways to do things like:
Before any treatment can be taken widely, potential new cancer medicines must be shown to be effective and relatively safe to take. As a result, researchers carefully track the experiences of people in clinical trials. This way, they can identify any safety problems or side effects, known as “adverse events.”
Clinical trials can take place in hospitals, universities, doctors' offices and community clinics. A trial might last weeks or years, depending on what is being studied and what the goals are. Participants are told how long the trial will last before they enroll. As telemedicine technology has become more available and improved, particularly because of the COVID-19 pandemic, certain patients that qualify can now be monitored remotely during their cancer clinical trials and for ongoing treatment.
Clinical Trial Participation Basics
Before someone can join a clinical trial, they must meet certain requirements. Factors that are needed for a person to participate are called “inclusion criteria,” while those that prevent someone from participating are called “exclusion criteria.” For example, a trial's inclusion criteria might require any participating patient to be diagnosed with breast cancer and be within a certain age range. Alternatively, a trial's exclusion criteria might not allow any patients who are pregnant. The requirements for each clinical trial relate to the questions the clinical trial is trying to answer.
The decision to participate must be completely voluntary. Anyone who decides to participate in a trial will be asked to provide written permission to be part of the study, known as “informed consent.” The process leading to informed consent helps educate a potential participant, in a language that the person understands, on all aspects and potential risks of the clinical trial they are about to enter to help them make their decision.
Several organizations and safety review boards around the world, such as the U.S. Food and Drug Administration (FDA) and the Office for Human Research Protections in the United States and the European Medicines Agency in Europe, protect patients in clinical trials by enforcing rules about how treatments are given and monitoring risks throughout the trial.
Phases of the Clinical Trial Process in Cancer
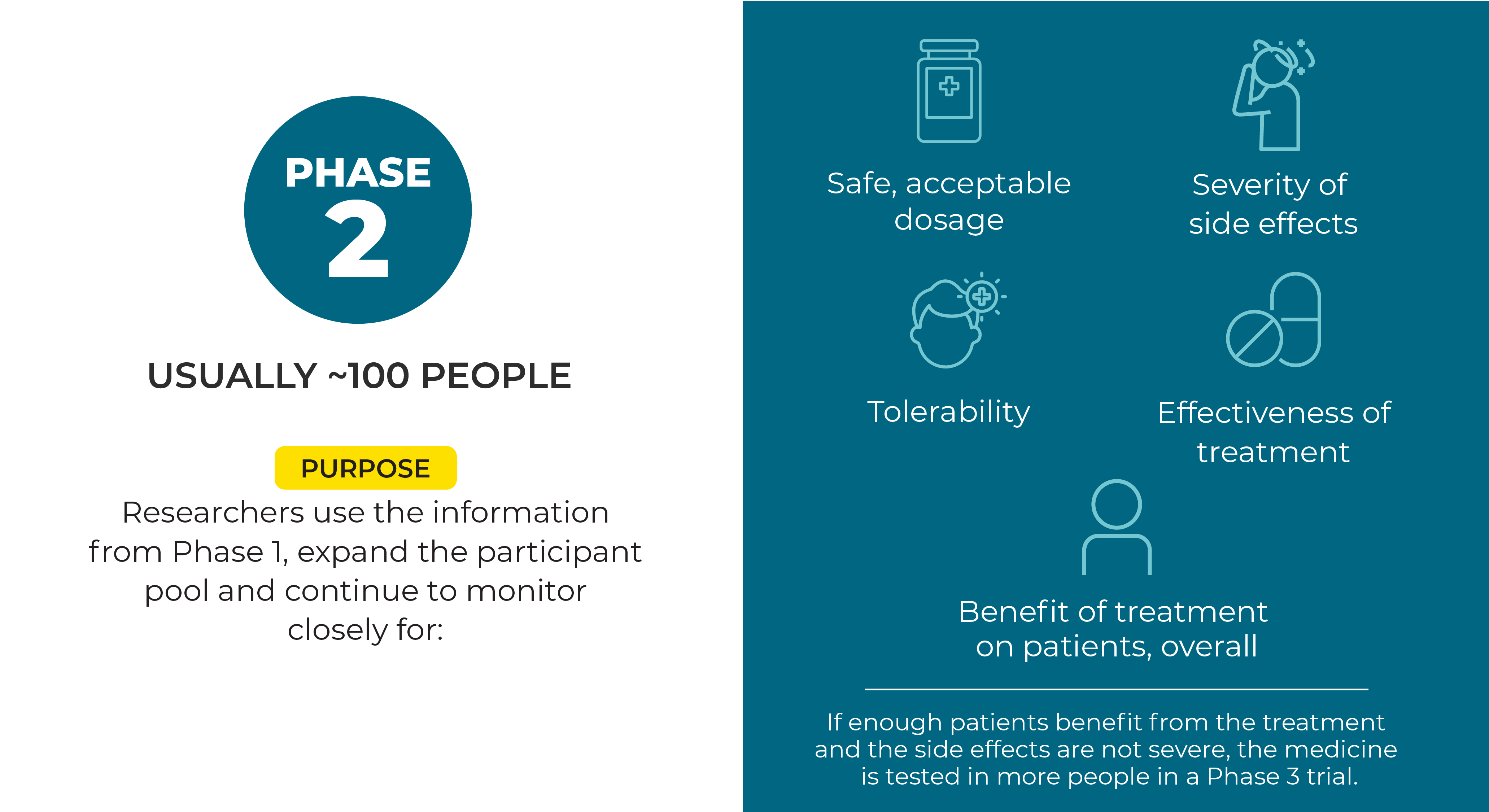
The testing of a potential new treatment involves a series of clinical steps, called phases. Each phase provides certain information about the treatment. If a potential new treatment is successful in one phase, then it can move on to more testing in the next phase. Knowing the phase of the clinical trial is important for participants because it provides insight into how much is known about the treatment being studied and what benefits and risks might be involved in taking part.
Randomized refers to when study participants are randomly assigned to either the new treatment or the treatment doctors currently use for that cancer. These groups of participants taking the different treatments make up the different “arms” of the study.
Sometimes, both the people participating in a Phase 3 trial and those running it do not know which treatment each participant is getting. This set-up is known as a “double-blinded” study. As with previous phases, people in Phase 3 clinical trials are monitored closely for side effects, and treatment is stopped if they’re harmful.
Since joining a clinical trial is voluntary, participants can opt to leave at any time for any reason, including side effects.
How Clinical Trial Success Is Measured
The goals of clinical trials are called endpoints, which are the results that show doctors if a medicine or treatment is working and its effects on the people taking the therapy. Endpoints might measure, for example, how long participants survive, whether their tumor shrank, how long their cancer is controlled, or the quality of their life. The FDA helps provide researchers with information on the best endpoints to use in their trials.
Here are a few common measures in clinical trials:
Clinical trials have had an immense impact on improving cancer care and providing knowledge to the scientific community. As a result, today, people are living longer than ever from successful cancer treatments. By participating in clinical trials, people living with cancer gain access to potential advancements in cancer care and help researchers discover new, more effective therapies. Most trials involve some form of risk to the participant, so asking important questions about the research, the risks, privacy and confidentiality, and other concerns, will help people get the information they need to decide whether to participate.